Adam Di Sarro blinded in one eye from contaminated eyedrops
A Florida firefighter has revealed that he lost vision in one eye after using eye drops laced with drug-resistant bacteria – and he has filed a lawsuit against the manufacturers.
Naples Fire Capt. Adam Di Sarro said he used EzriCare Artificial Tears for dryness in his left eye for several years without any problems until last fall, CBS News reported.
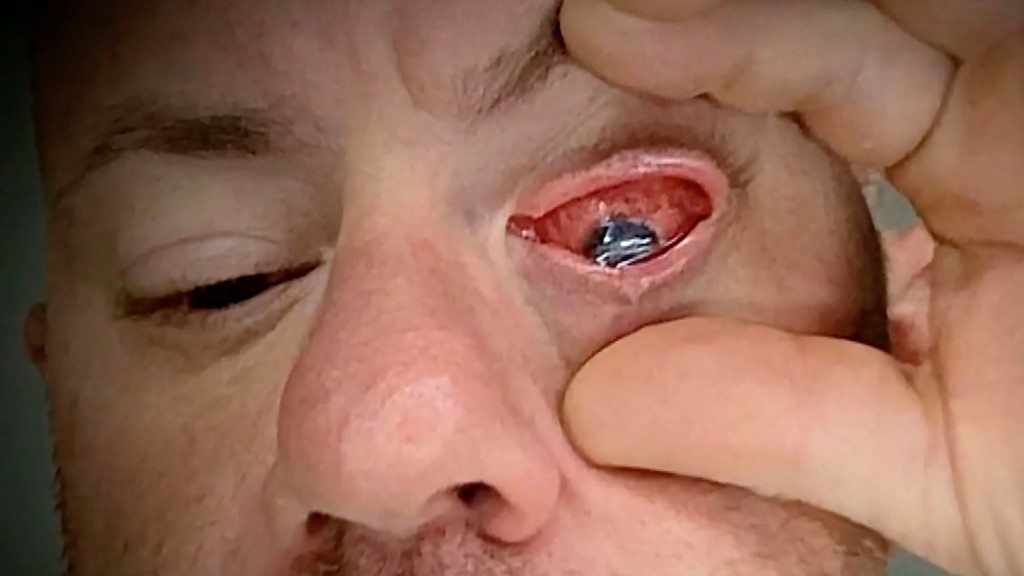
“The redness came on, the irritation came on, a lot of itching, and it was abnormal,” Di Sarro told the outlet. “It just progressively got worse, to the point where I couldn’t even see within a few hours.”
When antibiotics wouldn’t solve the infection, doctors feared he would lose his eye.
“That was hard and is still hard because I’m still not at work — going on five months,” Di Sarro said.

Dr. Guillermo Amescua, a cornea specialist at the Bascom Palmer Eye Institute of the University of Miami Miller School of Medicine, used an experimental light treatment that finally killed the infection, according to CBS News.
Di Sarro has filed a federal lawsuit in Florida against EzriCare and Delsam Pharma, which recently recalled batches after the over-the-counter drops were linked to cases in 16 states.
He also is suing Amazon, which distributed the eye drops. He has accused the manufacturers of negligence.
At least 68 people nationwide are known to have been infected by the contaminated drops in the outbreak of the antibiotic-resistant bacteria Pseudomonas aeruginosa.
Three people have died, eight blinded and four others needed an eyeball surgically removed.
The Centers for Disease Control and Prevention has identified cases in California, Colorado, Connecticut, Florida, Illinois, Nevada, New Jersey, New Mexico, New York, North Carolina, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin.
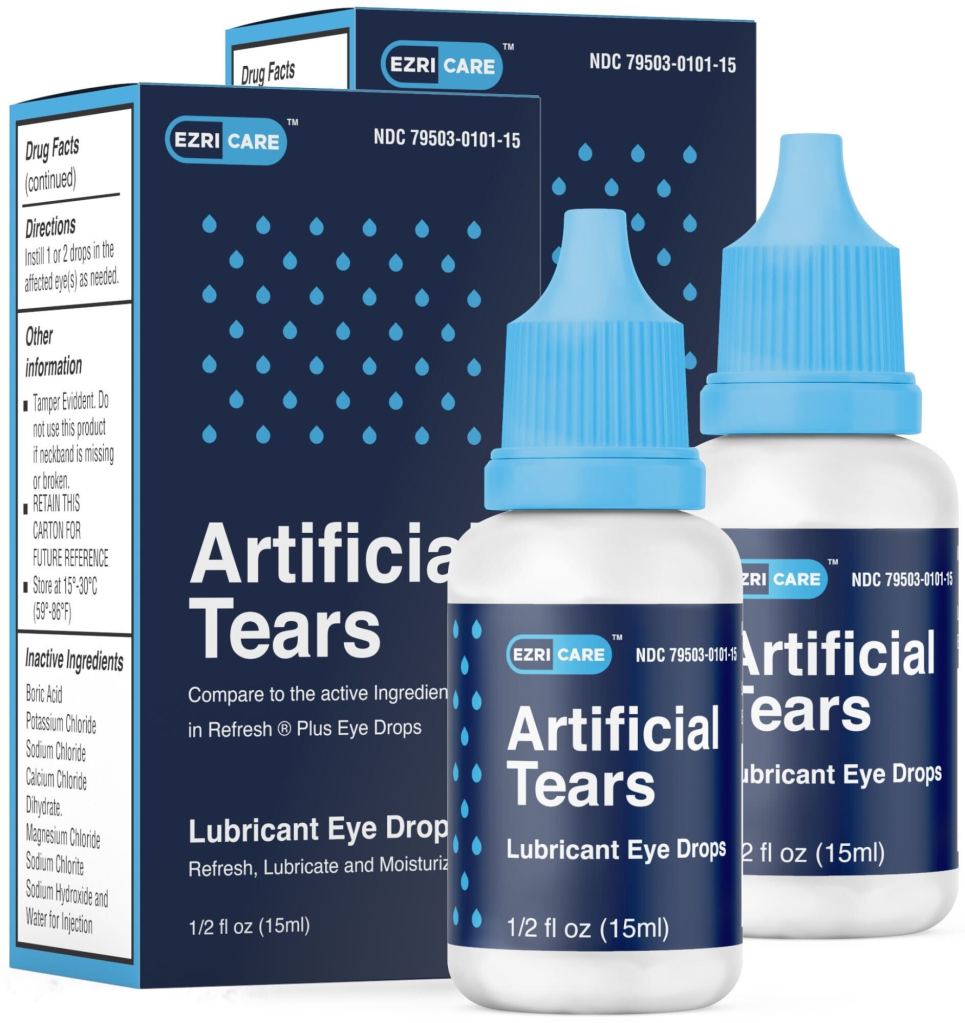
The agency noted that “patients reported over 10 different brands of artificial tears and some patients used multiple brands” — but EzriCare Artificial Tears “was the brand most commonly reported.”
“This was the only common artificial tears product identified across the four healthcare facility clusters,” the CDC said. “Patients and healthcare providers should immediately stop the use of EzriCare Artificial Tears.”
EzriCare and Amazon did not comment to CBS News. The Post has reached out to Delsam Pharma.
Read the full article Here


